Chemistry unit 1 review answer key – Get ready to ace Chemistry Unit 1 with our comprehensive answer key! This guide provides an engaging journey through the fundamentals of chemistry, making complex concepts accessible and exciting.
Dive into the world of matter, chemical reactions, stoichiometry, gases, and solutions, armed with clear explanations and practical examples. Our answer key empowers you to conquer every challenge and build a solid foundation in chemistry.
Introduction to Chemistry Unit 1
Unit 1 of chemistry introduces the fundamental concepts and principles that serve as the foundation for understanding the subject. It provides a comprehensive overview of the essential building blocks of matter and their interactions, laying the groundwork for subsequent units and the study of chemistry as a whole.
Key concepts covered in Unit 1 include:
Matter and Its Properties
- States of matter and their characteristics
- Physical and chemical properties of matter
- Classification of matter: elements, compounds, and mixtures
Atomic Structure, Chemistry unit 1 review answer key
- The structure of an atom, including the nucleus and electrons
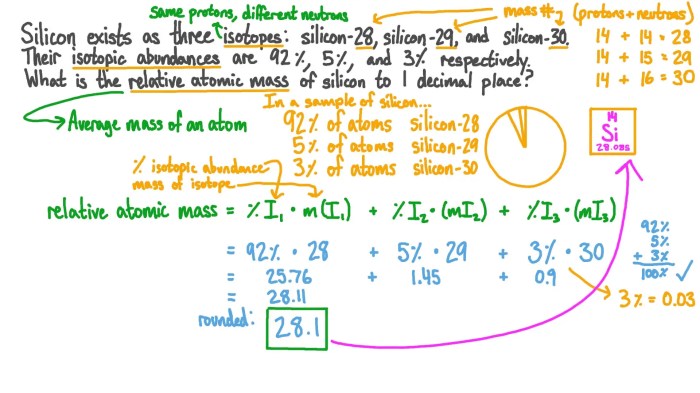
- Atomic number, mass number, and isotopes
- Electron configuration and the periodic table
Chemical Bonding
- Types of chemical bonds: ionic, covalent, and metallic
- Molecular geometry and bond polarity
- Intermolecular forces
Matter and Its Properties: Chemistry Unit 1 Review Answer Key
Matter is anything that takes up space and can be weighed. It exists in three states: solid, liquid, and gas. Solids have a definite shape and volume, liquids have a definite volume but no definite shape, and gases have no definite shape or volume.Matter
can be classified into two types based on its composition: elements and compounds. Elements are substances that cannot be broken down into simpler substances by chemical means. Compounds are substances that are made up of two or more elements that are chemically combined.
Mixtures are combinations of two or more elements or compounds that are not chemically combined.The physical properties of matter are those that can be observed without changing the composition of the matter. These properties include color, density, melting point, boiling point, and solubility.
The chemical properties of matter are those that describe how the matter reacts with other substances. These properties include flammability, reactivity, and toxicity.
Elements, Compounds, and Mixtures
Elements are the basic building blocks of matter. They are pure substances that cannot be broken down into simpler substances by chemical means. Each element is represented by a unique chemical symbol, such as H for hydrogen, O for oxygen, and Fe for iron.Compounds
are substances that are made up of two or more elements that are chemically combined. The elements in a compound are held together by chemical bonds. Compounds have different properties than the elements that make them up. For example, water is a compound made up of hydrogen and oxygen.
Water has different properties than hydrogen or oxygen alone.Mixtures are combinations of two or more elements or compounds that are not chemically combined. The components of a mixture can be separated by physical means, such as filtration or distillation. Mixtures have different properties than the pure substances that make them up.
For example, salt water is a mixture of water and salt. Salt water has different properties than pure water or pure salt.
Chemical Reactions
Chemical reactions are processes that involve the transformation of one set of chemical substances to another. They are fundamental to many natural phenomena and technological applications, such as combustion, digestion, and the production of materials.
Types of Chemical Reactions
- Combination reactionsoccur when two or more substances combine to form a single product. For example, when hydrogen and oxygen react, they form water: 2H 2+ O 2→ 2H 2O.
- Decomposition reactionsoccur when a single substance breaks down into two or more products. For example, when water is electrolyzed, it decomposes into hydrogen and oxygen: 2H 2O → 2H 2+ O 2.
- Single-replacement reactionsoccur when one element replaces another element in a compound. For example, when iron is added to a solution of copper sulfate, the iron replaces the copper in the compound, forming iron sulfate and copper: Fe + CuSO 4→ FeSO 4+ Cu.
- Double-replacement reactionsoccur when two compounds exchange ions to form two new compounds. For example, when sodium chloride and silver nitrate are mixed, they react to form sodium nitrate and silver chloride: NaCl + AgNO 3→ NaNO 3+ AgCl.
Factors Affecting Chemical Reactions
Several factors can affect the rate and extent of chemical reactions, including:
- Concentration of reactants: The higher the concentration of reactants, the faster the reaction will occur.
- Temperature: Increasing the temperature of a reaction generally increases the rate of the reaction.
- Surface area: Increasing the surface area of reactants increases the number of collisions between them, which can increase the rate of the reaction.
- Catalysts: Catalysts are substances that increase the rate of a reaction without being consumed in the reaction.
Stoichiometry
Stoichiometry is the branch of chemistry that involves the study of the quantitative relationships between reactants and products in chemical reactions. It helps us predict the amounts of reactants and products involved in a particular reaction and is essential for various applications in chemistry, such as predicting reaction yields, designing experiments, and optimizing industrial processes.
The Mole and Molar Mass
The mole is the SI unit of amount of substance, and it represents a specific number of elementary entities. One mole of a substance contains 6.022 × 10^23 entities, which can be atoms, molecules, ions, or electrons, depending on the substance.
The molar mass of a substance is the mass of one mole of that substance and is expressed in grams per mole (g/mol).
Stoichiometric Calculations
Stoichiometric calculations involve using the mole concept and balanced chemical equations to determine the quantitative relationships between reactants and products. These calculations allow us to predict the amount of reactants needed to produce a certain amount of product or the amount of product that can be obtained from a given amount of reactants.
Gases
Gases are a state of matter characterized by low density and high fluidity. They readily expand to fill their container and can flow easily. In this section, we will explore the properties, behavior, and laws governing gases.
Gases exhibit several distinctive properties:
- Low density:Gases have significantly lower densities compared to solids and liquids.
- High fluidity:Gases flow easily and can move around obstacles without significant resistance.
- Expansion:Gases expand to fill the volume of their container, taking the shape of the container.
- Diffusion:Gases mix with each other and spread throughout the available volume.
The behavior of gases can be explained using the kinetic molecular theory, which states that gases are composed of tiny particles (molecules or atoms) that are in constant random motion. These particles collide with each other and with the walls of their container, exerting pressure on the container.
The pressure of a gas is defined as the force exerted by the gas per unit area. Volume refers to the space occupied by the gas, and temperature measures the average kinetic energy of the gas particles. These three properties are related by the ideal gas law:
PV = nRT
where P is pressure, V is volume, n is the number of moles of gas, R is the ideal gas constant, and T is temperature.
The ideal gas law can be used to solve problems involving gas properties. For example, if the pressure of a gas is increased while the temperature and number of moles remain constant, the volume will decrease. Similarly, if the temperature of a gas is increased while the pressure and number of moles remain constant, the volume will increase.
Solutions
A solution is a homogeneous mixture of two or more substances. The substance present in the largest amount is called the solvent, while the other substances are called solutes. Solutions can be classified into different types based on the physical state of the solvent and the solute.
The concentration of a solution refers to the amount of solute present in a given amount of solvent or solution. There are several ways to express the concentration of a solution, including molarity, molality, and mass percentage.
Factors Affecting the Solubility of Solutes
The solubility of a solute in a solvent depends on several factors, including:
- Temperature:The solubility of most solids and liquids increases with increasing temperature. However, the solubility of gases decreases with increasing temperature.
- Pressure:The solubility of gases increases with increasing pressure. This is because gases are more soluble in liquids under higher pressure.
- Nature of the solute and solvent:The solubility of a solute in a solvent depends on the chemical nature of both substances. In general, polar solutes are more soluble in polar solvents, and nonpolar solutes are more soluble in nonpolar solvents.
General Inquiries
What topics are covered in Chemistry Unit 1?
Matter and its properties, chemical reactions, stoichiometry, gases, and solutions.
How can I use this answer key effectively?
Check your answers, identify areas for improvement, and reinforce your understanding of key concepts.
Is this answer key suitable for all students?
Yes, it’s designed to support students of all levels, from beginners to advanced learners.