Isotopes and average atomic mass worksheet answers – Embark on an enlightening journey into the realm of isotopes and average atomic mass, where the very foundations of chemistry are unveiled. This comprehensive guide, tailored to the accompanying worksheet, unravels the mysteries of atomic structure, providing a profound understanding of the fundamental principles that govern the behavior of matter.
Delving into the intricate world of isotopes, we explore their profound impact on atomic mass. Through meticulously crafted examples, we elucidate the techniques employed to calculate the average atomic mass of elements, empowering you with the tools to navigate the complexities of atomic diversity.
Isotopes and Atomic Mass: Isotopes And Average Atomic Mass Worksheet Answers
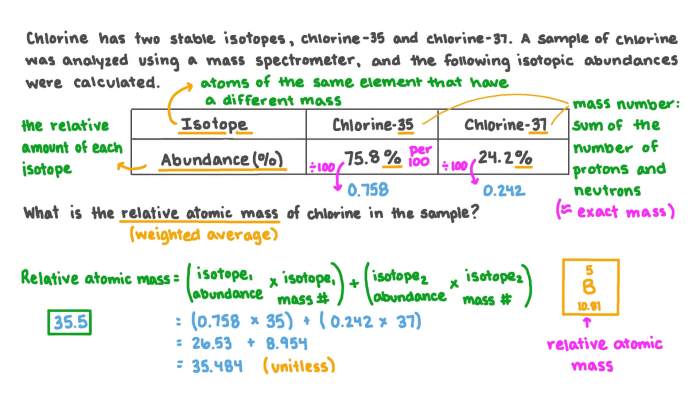
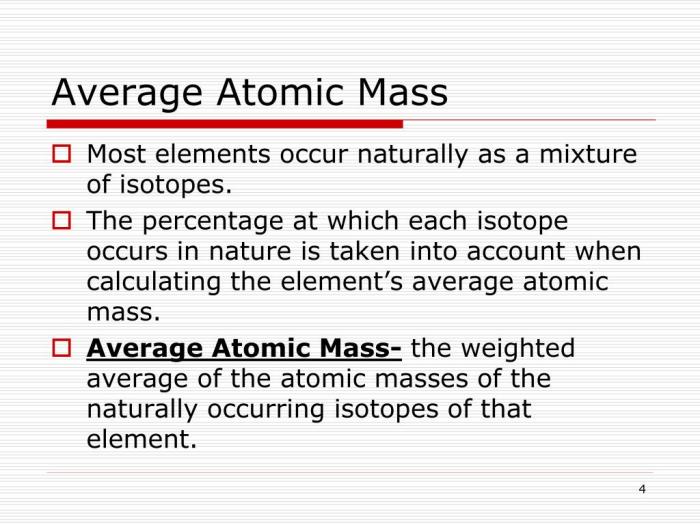
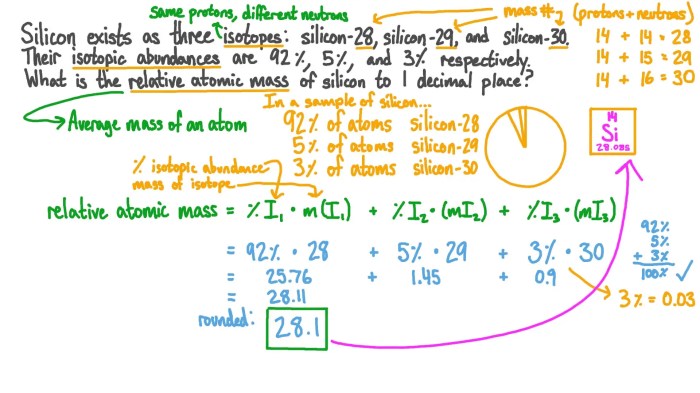
Isotopes are variations of the same element that have the same atomic number but different neutron numbers. This difference in neutron number results in different atomic masses for the isotopes of an element. The atomic mass of an element is the weighted average of the atomic masses of its isotopes, taking into account their relative abundances.
For example, chlorine has two stable isotopes: chlorine-35 and chlorine- 37. Chlorine-35 has 17 protons and 18 neutrons, while chlorine-37 has 17 protons and 20 neutrons. The atomic mass of chlorine is calculated as follows:
“`Atomic mass = (abundance of chlorine-35
- atomic mass of chlorine-35) + (abundance of chlorine-37
- atomic mass of chlorine-37)
“`
Given that chlorine-35 has an abundance of 75.77% and chlorine-37 has an abundance of 24.23%, the atomic mass of chlorine is:
“`Atomic mass = (0.7577
- 34.969) + (0.2423
- 36.966) = 35.453
“`
Worksheet Analysis
The worksheet on isotopes and average atomic mass provides practice in calculating the average atomic mass of elements with multiple isotopes. The worksheet includes problems involving:
- Calculating the average atomic mass of an element given the masses and abundances of its isotopes
- Using the periodic table to find the atomic masses of isotopes
- Applying the concept of isotopes to real-world examples
Problem-Solving Techniques
To solve problems related to isotopes and average atomic mass, follow these steps:
- Identify the isotopes of the element and their atomic masses.
- Determine the relative abundances of the isotopes.
- Use the formula for average atomic mass: Atomic mass = (abundance of isotope 1
- atomic mass of isotope 1) + (abundance of isotope 2
- atomic mass of isotope 2) + …
- Calculate the average atomic mass by multiplying the atomic mass of each isotope by its abundance and summing the results.
For example, to calculate the average atomic mass of magnesium, which has three isotopes with atomic masses of 24.305, 25.307, and 26.305 and abundances of 78.99%, 10.00%, and 11.01%, respectively:
“`Average atomic mass = (0.7899
- 24.305) + (0.1000
- 25.307) + (0.1101
- 26.305) = 24.307
“`
Applications of Isotopes
Isotopes have a wide range of applications in various fields, including:
- Medicine:Isotopes are used in medical imaging, such as X-rays and PET scans, and in cancer treatment, such as radiotherapy.
- Industry:Isotopes are used in quality control, materials testing, and tracing the flow of materials in industrial processes.
- Environmental science:Isotopes are used to study environmental processes, such as water movement and pollution, and to date archaeological and geological samples.
Isotopes offer unique advantages over other elements or techniques due to their specific properties and behavior, such as their ability to be traced and their distinctive chemical and physical characteristics.
Quick FAQs
What is an isotope?
An isotope is a variation of an element with the same atomic number but a different number of neutrons, resulting in a distinct atomic mass.
How is average atomic mass calculated?
Average atomic mass is calculated by multiplying the mass of each isotope by its abundance and summing the products.
What are the applications of isotopes in medicine?
Isotopes are used in medical imaging techniques, such as PET scans, to diagnose and monitor diseases.